
Advantages of IKA electrochemistry
Electrochemistry or electrochemical engineering is a technology that has been around for about 150 years and for certain processes it is the “golden standard". In the past however, today's conventional methods had clear advantages over electrochemistry.
That all changed with the development of new electrode materials and recent research in electro-organic synthesis. With electrochemistry, production is faster, more effective, cleaner and more cost-effective. When regenerative energy is used, even "green chemistry" is possible.
IKA offers flow electro systems and batch systems for electrochemical process engineering. In flow electric systems, the reagents are pumped through a flow cell. When the reagent meets the electrodes in the flow cells, the electrochemical reaction takes place.
With the ElectraSyn flow system, applications such as these are possible:
- Oxidation of bromide, iodide, benzyl, furan
- Oxidation of aromatic compounds
- Quinone reduction
- Dehalogenation
- Hydrogen production
- Deoxygenation
- Reduction of CO2 and nitro compounds
Electro-Organic Synthesis Systems
Downloads
Applications
Nitrile synthesis
|
Nitrile synthesis |
|
|
|
|
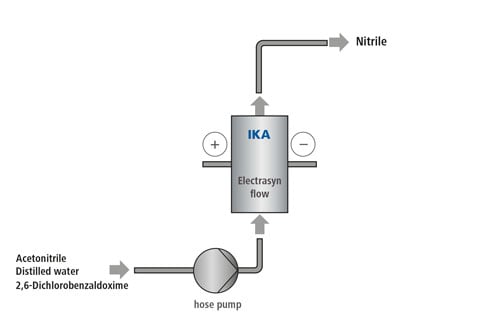
55 ml of acetonitrile are mixed with 5 ml of distilled water in a suitable vessel and then 1.38 g (7.26 mmol) of 2.6 dichlorobenzaldoxime is stirred in. The solution must be absolutely free of solid components before being pumped into the system. The structure of the cell system begins with the cell: A half cell with graphite electrode is used as the anode. On the cathode side, a lead-bronze electrode is used. When the cell is completely assembled, the stand, hoses, power supply and pump are connected accordingly. The current density in this synthesis is 5 mA/cm2, which corresponds to a current of 60 mA. The voltage can be set to the maximum, as it automatically adjusts to the electrical resistance of the system. The pump is set to a flow rate of 8.5 ml/min. or 0.142 ml/sec. The product leaving the cell differs from the reagent since it is slightly yellow. Further information on the preparing and processing the product solution can be found at: C. Gütz, A. Stenglein, S. R. Waldvogel, Highly Modular Flow Cell for Electro-Organic Synthesis, Org. Process Res. Dev. 2017, 21, 771–778. [DOI: https://pubs.acs.org/doi/abs/10.1021/acs.oprd.7b00123 ] |
|
